Is ORP Really Your Best Option for Dechlorination Measurement?
Low-level Chlorine Detection
With multiple variables of pH, temperature, oxidants, and reductants all contributing to an oxidation reduction potential (ORP) value, trying to identify low levels of chlorine in the water can become a bit of a guessing game. Here are some considerations for achieving more reliable accuracy when quantifying dechlorination results for applications ranging from RO membrane protection to wastewater discharge compliance.
Chlorine’s Complicated Relationship With ORP
Unlike direct chlorine measurement methods — such as DPD colorimetric — ORP readings have an inferred relationship with the amount of chlorine in a flow of water, one that can be complicated by other variables. ORP readings may address the question “Have we eliminated all the chlorine possible to protect our RO membranes or meet our wastewater discharge requirements?” by encouraging overfeed of dechlorinating agents. However, ORP would not be useful to ensure chlorine residuals at a parts-per-billion (ppb) level providing enough disinfection to prevent excessive biogrowth while not damaging the membranes. That is where RO applications that depend on a chlorination/dechlorination process to maintain this delicate balance can benefit from accuracy of the ULR CL17sc, colorimetric DPD analyzer.
When making chlorination/dechlorination decisions, it is important to understand ORP measurement technology — the difference in electrical potential (expressed in millivolts) between a measurement electrode exposed to the solution being evaluated and a reference electrode immersed in a concentrated salt solution. In water samples with controlled temperature and pH, and with chlorine as the only oxidant, the positive electric potential measured by the ORP probe can be correlated to the concentration of chlorine. Unfortunately, that inferred relationship could be complicated by a variety of extraneous factors that can impact the ORP reading, create a risk of the RO membrane damage, or cause an exceedance of chlorine limits in wastewater discharge, due to the following.
- Water Conditions: Because ORP is an electrochemical measurement used to infer the chlorine level, this inferred relationship can be affected by a number of variables. For example, as the pH reading increases, the ORP reading will fall. ORP readings may also be affected by water temperature, which in many applications is difficult to maintain constant, as well as, by changes in sample flow and pressure.
- Other Oxidants/Reductants: Since ORP is measuring the difference in electrical potential of the sample and a standard reference, any oxidants (not just chlorine) and any reductants (or reducing agents) can impact the numerical ORP reading thus affecting the correlation to the chlorine concentration in the water.
- ORP Non-Linear Response: ORP sensors usually respond quickly to an increase of oxidants in the water, allowing for a fast reaction to those situations, such as a lapse in addition of sodium bisulfite (SBS). However, their response to overfeed of the reducing agent and restoring of chlorine absence is usually very slow.
By contrast, a direct chlorine analysis provided by colorimetric DPD technology makes it possible to detect and measure total chlorine accurately, regardless of other circumstances or changes in the water.
How ORP Misinterpretations Can Hurt
Any misperception that ORP measurements only respond to free chlorine selectively can be a cause for misguided chlorination/dechlorination decisions. Because ORP is not designed to measure chlorine levels directly — but rather the balance between the presence of any oxidants (taking on electrons) and/or reductants (giving up electrons) — other factors can come into play. A misleading low ORP reading can be misinterpreted and true chlorine concentrations may damage RO membranes and shorten their life. On the other hand, if it is incorrectly assumed that chlorine is the primary cause of a high ORP number, overdosing a scavenger such as SBS can eliminate dissolved oxygen, creating an anaerobic environment. That, combined with the resulting higher levels of sulfite supporting anaerobic bacterial growth, can exacerbate biofouling on RO membrane surfaces.
Case Study 1
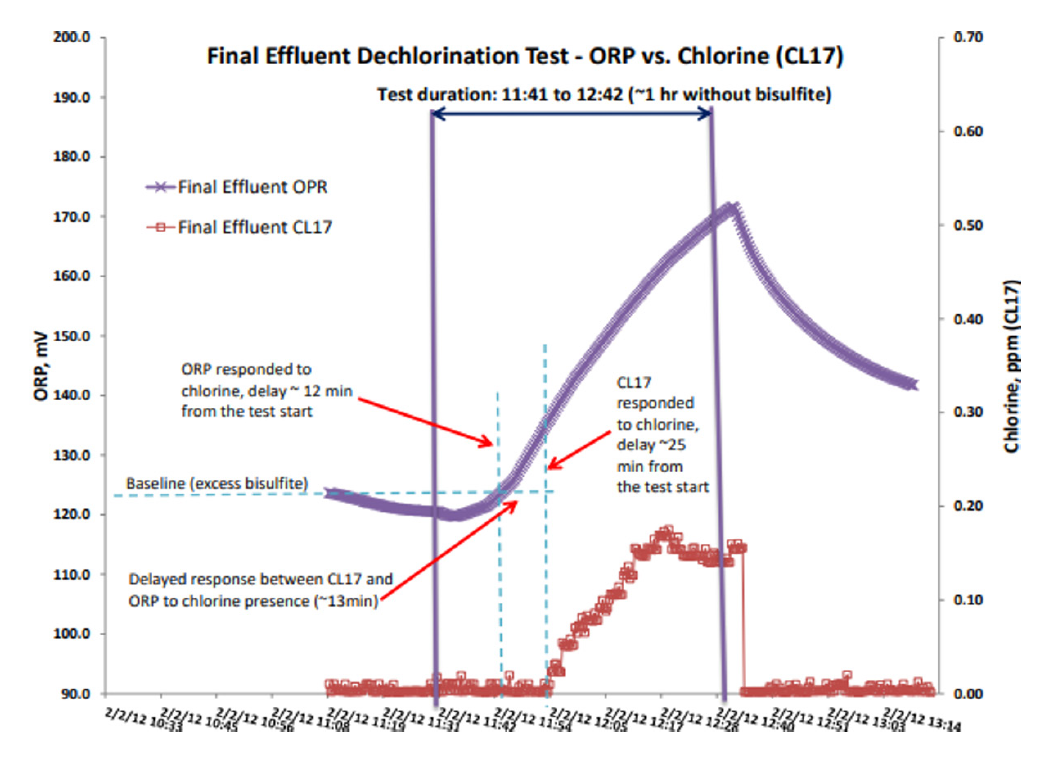
A comparative test was conducted at a Wastewater Treatment Plant employing chlorination/dechlorination of final effluent before discharge. This test provided comparison between response time to chlorine presence and absence for colorimetric sensors (examples: CL17 and ULR CL17sc) vs ORP sensors.
Figure 1 illustrates that ORP provides relatively fast response to chlorine breakthroughs (only about 12 minutes faster than the CL17). However, ORP’s response to excess of reducing agents, like Sodium Bisulfite (SBS) in this test, can be much longer than of the CL17 (Fig. 1). Moreover, relying on absolute values of ORP can be misleading due to limitations of this technology and its relative nature. Correlating ORP and chlorine concentration to quantify the response may lead to severe problems, regardless of the ORP sensors, because ORP technology provides only a surrogate for chlorine measurement.
Case Study 2
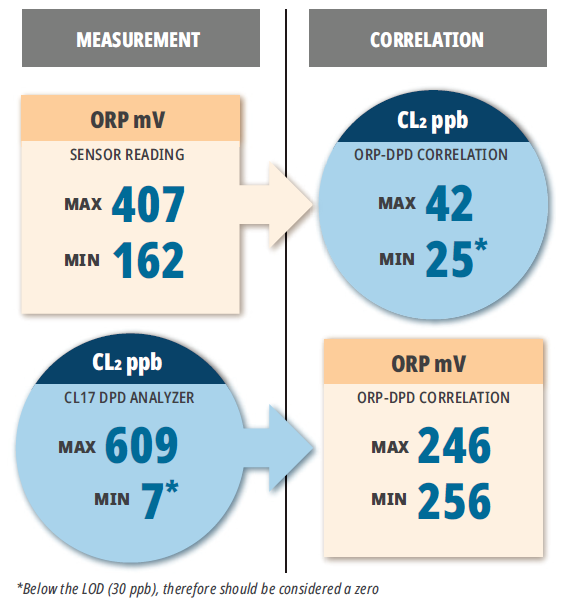
A test at electronic chip manufacturing facility was conducted in UPW preparation cycle involving dechlorination of city water with SBS. ULR CL17sc chlorine analyzer was added to the suite of a regular CL17 and an ORP sensor. Prior to the trial, the facility personnel constantly tried to correlate the readings of their CL17 and the ORP probe. The analysis of the time-stamped data showed that such correlation could not be used for process control, Figure 2.
As follows from Figure 2, maximums and minimums of the ORP readings recorded in the logs did not match the max/min chlorine concentrations registered at the same time. This observation highlights how dangerous it could be to rely solely on ORP readings to control dechlorination process. This example explains why so many dechlorinating facilities overfeed SBS and highlights an old wisdom proclaiming that “you cannot control what you do not measure” while adding: measure “accurately”.
Conclusion
Based on the conducted studies it becomes clear that ORP sensing technology cannot provide adequate and stable correlation with chlorine concentration in applications when water conditions may change. ORP sensors may serve to detect breakthrough of oxidants in the water quickly, as long as its pH is stable, at a minimum. Moreover, the response of ORP sensors to overfeed of the reducing agents may be long and sluggish.
At the same time, DPD colorimetric analysis provides accurate detection of both excess of oxidant, e.g. chlorine, or reductant, e.g. bisulfite. As seen from the conducted testing, online colorimetric analyzers can react to chlorine breakthrough comparatively fast and will immediately detect excess of bisulfite, allowing to prevent significant overfeed of chemicals providing direct cost savings.
Additional Resources
Pairing Digital Flow Sensors with the Hach AS950 Automatic Sampler
go to HACH.COMIntroduction When the Hach® AS950 Automatic Sampler launched in 2015, we were excited to offer the option to connect digital flow sensors to the controller. Digital flow sensors can be used for collecting flow proportional samples, as well as triggering...

Maximizing Phosphorus Removal
go to HACH.COMPhosphorus removal plays a critical role in protecting waterways, meeting permit requirements, and managing treatment costs. But optimizing phosphorus treatment isn’t always straightforward, especially when processes, influent conditions, and system...

Optimize Total Suspended Solids (TSS) & Turbidity Measurement
go to HACH.COMAccurate measurement of Total Suspended Solids (TSS) and turbidity is vital for effective water quality management across municipal, industrial, and wastewater environments. Real-time insights into solids concentrations empower operators to improve...
Privacy Policy | Cookie Policy | Cookie Settings | Do Not Sell or Share My Data
©Hach All rights reserved.




