CL17 for Permanganate Measurement in Drinking Water
Introduction
Two of the most abundant metals in the Earth’s crust are iron and manganese; these metals are also found in ground and surface waters. The effects of elevated levels of iron and manganese have been discussed in drinking-water-treatment-related as well as regulatory literature and may be summarized as causing aesthetic problems in the distribution system such as staining and bad taste and odor. Although these metals have no adverse health effects, it is recommended that their levels be reduced to below SMCL established by the U.S. EPA.
The main goal of water treatment with sodium permanganate or potassium permanganate is to provide adequate pre-oxidation to remove organics and/or dissolved metals, such as manganese and iron, from the water. Natural organic matter (NOM) removal is usually the primary goal for surface water treatment to minimize formation of disinfection byproducts (DBPs). Removal of metals by oxidation is usually the primary goal for ground water treatment; however, the general practice is to sequentially remove metals by precipitation and filtration.
Operational challenges usually come to determining and maintaining the correct feed of permanganate. The balancing act is between underfeeding permanganate and therefore not achieving the goal of NOM and/or metals removal and overfeeding that may lead to an unwanted pink color in finished water. In addition, over-oxidizing of Mn leads to its dissolution and therefore defeats the purpose of the treatment (inefficient filtration). To maintain such delicate balance, the analysis of treated water must be constantly conducted in process and therefore on-line instrumentation is preferred for such applications. Instrumentation should provide accurate results and be robust.
Measuring Permanganate with the CL17
To understand the Hach CL17 chlorine analyzer capabilities for permanganate analysis, several tests were conducted, both in the laboratory and in the field. Total chlorine chemistry was selected to use in testing as the formulation of these reagents provides better accuracy. The main goals of laboratory testing were to verify the linearity of response to permanganate, validate accuracy/precision specifications, and evaluate main interferences, including chlorine.
The graph presented in Figure 1 illustrates linear response of two CL17 analyzers with total chlorine reagents to an array of KMnO4 (potassium permanganate) standard solutions within the range of 0 – 5 ppm. It also illustrates the dispersion of the results (2.38%), which defines accuracy of the measurement across the range.

Based on the laboratory testing, it was concluded that the CL17 will linearly respond to permanganate in water and the major analytical performance specifications were derived from the laboratory test results according to the EPA guidelines. Additional testing was conducted to evaluate the influence of several potential interferences usually present in source water.
Test results are presented in the table below.
Laboratory Test Results
| Specification | Result |
|---|---|
| Measurement Range | 0–5 ppm, as Potassium Permanganate |
| Precision | 5% or 0.03 ppm, whichever is greater |
| Accuracy | ±10% or 0.05 ppm, whichever is greater |
| Linearity | Existing CL17 calibration curve1 |
| Total Hardness (>1000 ppm) | Negative interference, 10% at 1000 ppm |
| Total Alkalinity (>300 ppm) | Negative interference, 7% at 300 ppm, 40% at 1000 ppm |
| Total Acidity (≤150 ppm) | None |
| Chlorine | Positive interference, 100% at all levels2 |
A special test was conducted to evaluate CL17 measurement of combined oxidants in the sample – permanganate and chlorine present simultaneously. The test revealed the CL17 reads a linear sum of the components’ concentrations; no specific interaction between the components was detected. The positive results of the laboratory testing instilled confidence to proceed with a field study that was conducted the Hummelstown drinking water treatment plant (DWTP), a facility managed by United Water in Pennsylvania.
Field Study
The Hummelstown DWTP has used permanganate for pre-oxidation and chlorine for post-chlorination for the past several years. The main idea of such treatment is to control NOM in the source water to minimize formation of DBP. The plant treats surface water coming from a creek with membrane filtration, including pre-oxidation with permanganate followed by primary disinfection with chlorine and post-chlorination after the filters.
The main challenge that facility personnel face arises from the variability of their source water quality. Source water has variable chlorine demand comprised of NOM/bacterial load and undergoes seasonal changes in iron and manganese. Pre-oxidation with KMnO4 takes care of the metals and helps to reduce some of the NOM. In addition, the rest of the NOM as well as bacterial load is reduced by sequential chlorine addition (purchased 12.5% hypochlorite solution) prior to membrane filtration.


A CL17 analyzer equipped with total chlorine reagents was installed to sample water at approximately 1000 feet after the permanganate injection point to quantify its residual concentration prior to sequential addition of chlorine. The data collected from this CL17 were compared to the feed rate and theoretical permanganate concentration. Testing was conducted during the last week of March 2015 to achieve the desired water temperature and demand. The collected results presented a challenge for analysis due to cyclical nature of the plant’s operation caused by varying flow demand and corresponding control. Therefore, due to the flow dependencies and some natural gaps in the test procedure, a trend analysis of measured and calculated residuals was conducted (Fig. 3).
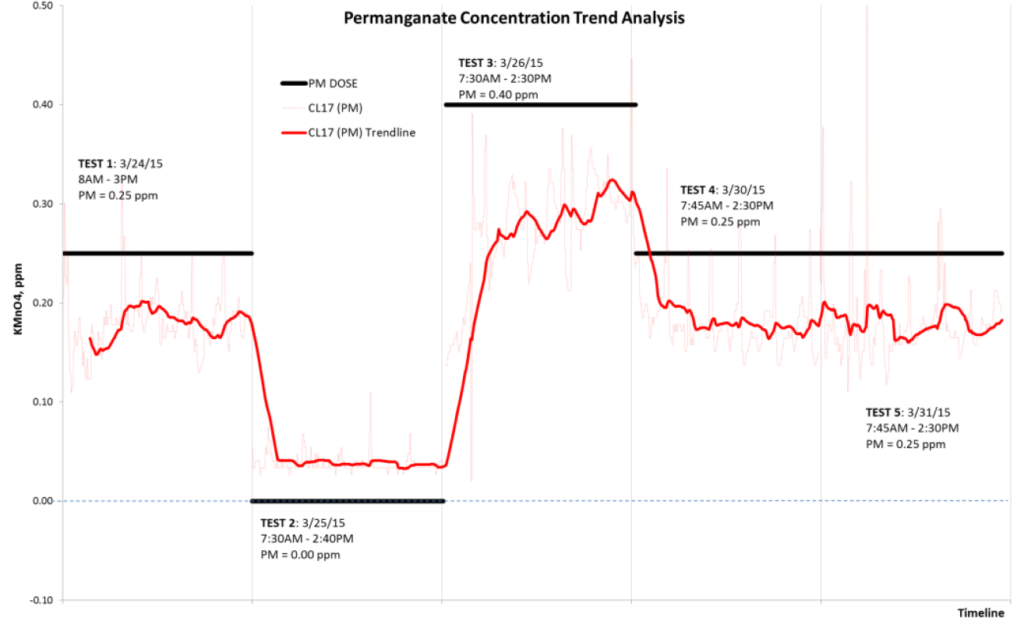
Field Study Results
| Test | Date/Time | KMnO4 Dose (ppm) | CL17 Average (ppm) | Oxidant Demand (ppm) | Oxidant Demand (%) |
|---|---|---|---|---|---|
| 1 | 3/24/15, 8:00–15:00 | 0.25 | 0.18 | 0.07 | 28% |
| 2 | 3/25/15, 7:30–14:40 | 0 | 0.043 | NA | NA |
| 3 | 3/26/15, 7:30–14:30 | 0.40 | 0.27 | 0.13 | 33% |
| 4 | 3/30/15, 7:45–14:30 | 0.25 | 0.18 | 0.07 | 28% |
| 5 | 3/31/15, 7:45–14:30 | 0.25 | 0.18 | 0.07 | 28% |
As seen from the Figure 3 and Table 2, the measured permanganate residual correlated with the expected. Permanganate demand was observed to be quite stable (~0.07 ppm or 28%) during all tests; however, for Test 3 the permanganate demand increased. In this test the permanganate feed was also increased by 60% and its residual increased by approximately 50%, so the discrepancy was about 10%. This value, especially with regard to the difference in absolute values of calculated demand (0.06 ppm) cannot be considered significant given the established accuracy specification.
Conclusions & Recommendations
- The conducted laboratory and field studies demonstrated analytical capabilities of the Hach CL17 analyzer to measure permanganate concentration in water.
- Practical application of the CL17 analyzer measuring permanganate residual in water sample was confirmed as a robust solution and good tool for control of challenging water treatment processes (variable water quality and demand).
- The obtained results clearly indicated that the suggested application of the CL17 analyzer can also be used for analytical determination of permanganate residual and that further testing at another facility with more stable water conditions (e.g. water temperature and chlorine demand) should be conducted to further prove such an approach.
Key Outcome: The Hach CL17 analyzer provides reliable, on-line monitoring of permanganate residuals, helping utilities balance treatment effectiveness, minimize overfeeding, and maintain water quality compliance.
| Challenge | CL17 Advantage |
|---|---|
| Risk of underfeeding, leaving organics/metals untreated | Accurate detection ensures effective oxidation |
| Risk of overfeeding, causing pink water and Mn dissolution | Reliable monitoring prevents overdosing |
| Seasonal NOM, iron, and manganese variability | Continuous data supports real-time process adjustments |
| Need for robust, low-maintenance instrumentation | CL17 proven stable in field studies, easy to operate |
Acknowledgement
Hach Company would like to express deep gratitude to the Hummelstown DWTP staff and personally to Mark Baker and Chad Bingaman for their support of the test.
Additional Resources
Pairing Digital Flow Sensors with the Hach AS950 Automatic Sampler
go to HACH.COMIntroduction When the Hach® AS950 Automatic Sampler launched in 2015, we were excited to offer the option to connect digital flow sensors to the controller. Digital flow sensors can be used for collecting flow proportional samples, as well as triggering...

Maximizing Phosphorus Removal
go to HACH.COMPhosphorus removal plays a critical role in protecting waterways, meeting permit requirements, and managing treatment costs. But optimizing phosphorus treatment isn’t always straightforward, especially when processes, influent conditions, and system...

Optimize Total Suspended Solids (TSS) & Turbidity Measurement
go to HACH.COMAccurate measurement of Total Suspended Solids (TSS) and turbidity is vital for effective water quality management across municipal, industrial, and wastewater environments. Real-time insights into solids concentrations empower operators to improve...
Privacy Policy | Cookie Policy | Cookie Settings | Do Not Sell or Share My Data
©Hach All rights reserved.