Nitrification Optimization in the Real World at Grapevine, TX, WWTP
Many watersheds in the US are susceptible to Nitrogen and Phosphorous nutrient pollution. Forms of phosphorus and nitrogen are therefore often top the list of key pollutants which are regulated and can also present significant treatment complexities. Water body impairment and aquatic environmental stewardship requires data. Data on what pollutants may impair receiving waters for their designated uses such as primary or secondary contact and to protect sensitive aquatic life. Testing and monitoring point and non-point discharges to receiving waters is done to understand the impact of pollutants for each watershed. Data also drives wastewater treatment effectiveness and optimization. Grab sample and composite data is required for permit compliance but when biological treatment processes like Nitrification are used, maximum treatment effectiveness and optimization requires something more.
With both permit compliance and plant optimization as driving factors, this note will cover how one facility in Texas achieved both. The Grapevine Wastewater Treatment plant ran a trial using an innovative technology from Hach® achieving up to a 28% reduction in energy use from their standard operation.
Maintaining consistent permit compliance for nutrients requires higher levels of attention and data for those biological systems than other types of pollutants. Nitrogen in the form of ammonia is most often the primary regulated nutrient for water quality. Many wastewater treatment plants across the globe are required to monitor ammonia nitrogen concentrations for permit compliance.
Regulations for water quality can include nitrogen species such as: Total Nitrogen (TN), Total Kjeldahal (TKN), Ammonia (NH3), Nitrite (NO2) and Nitrate (NO3). This note will primarily cover the most common nitrogen species given to wastewater facilities as a numerical regulation – Ammonia. Ammonia nitrogen can be a standalone nitrogen species as a permit limit or it can be part of a larger Nitrogen form as Total Nitrogen, Total Inorganic Nitrogen and or Total Kjeldahl Nitrogen as shown above in figure 1.
Nitrification is the key biological process to remove ammonia. Ammonia is removed aerobically in oxic zones where microorganisms convert it primarily to Nitrite and Nitrate. Controlled nitrification within wastewater treatment plants is usually the single most energy consumptive process at the plant. Adding air to water is commonly achieved with either sub surface air addition using blowers or surface aeration using mechanical water agitation.
In addition to the high energy use to achieve proper nitrification it is also a very sensitive system to diurnal load changes, seasonal temperature fluctuations, toxic loads and biological solids imbalances. Any of these conditions, or combination of issues, can cause extremely rapid deterioration in effluent water quality and the possible loss of nitrification. Sometimes these conditions can be fixed quickly such as under aeration, when system air flow can be increased, but in other cases loss of nitrification can take days to recover.
There are two commonly used forms of nitrogen that are important to wastewater treatment which can be confused, ammonia and ammonium.
| Total Nitrogen (TN) | ||||
|---|---|---|---|---|
| Total Inorganic Nitrogen (TIN) | ||||
| Particulate Organic – N |
Soluble Organic – N |
Ammonia – N (NH₃–N) |
Nitrite – N (NO₂–N) |
Nitrate – N (NO₃–N) |
| Total Kjeldahl Nitrogen (TKN) | ||||
Ammonia and Ammonium
Is it ammonia or ammonium? It depends. It depends on pH and temperature. The atomic weight between ammonia and ammonium varies by one hydrogen atom and the prevalence of either species is pH dependent. At a pH of 9.25 at 25°C, the prevalent species will be half ammonia (NH3) and half ammonium (NH4+). Typical municipal wastewater pH is commonly between 6.9 and 8.2. In most municipal wastewater systems, the predominant form found is ionic, measured as ammonium NH4+. This is of course dependent on the wastewater pH and temperature at each measurement location. This conditional relationship is illustrated in figure 2.
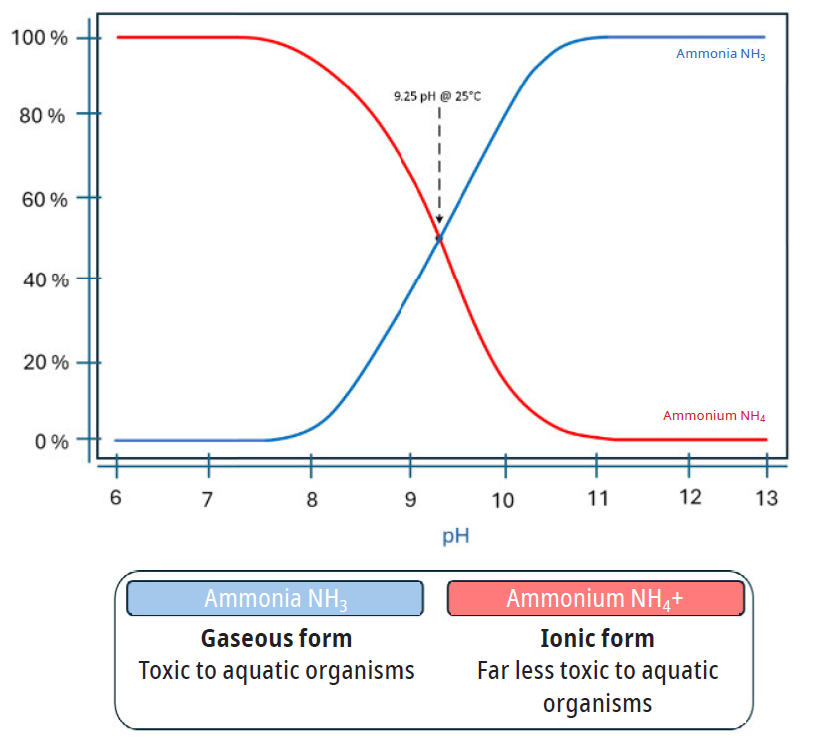
Ammonium NH4+ is the ionic form and considered far less toxic to aquatic organisms.
Ammonia NH3 is gaseous form, is considered highly toxic to aquatic organisms and is the form on most wastewater permits.
Gas sensing ammonia technology from Hach is an industry workhorse for online ammonia monitoring and control. This technology used at the Grapevine Wastewater treatment plant, described below, uses a simple and fast principle for measuring ammonia in this dynamic environment of pH and temperature. The analyzer titrates the sample over 12 pH and then measures the gaseous ammonia (NH3) concentration. This innovative methodology creates the fastest measurement cycle of any online wet chemistry based analyzer and allows for confidence no matter the pH or temperature, a true value of ammonia is being measured. The gas sensing method also removes any concern of interference from color in the sample that can be an issue with colorimetric methods.
After the analyzer completes conversion of the ammonium ions to ammonia gas the measurement value is displayed as ammonium (NH4+).
City of Grapevine TX, Background
The Grapevine Texas city municipal wastewater system serves a population of about 51,000 and discharges into a tributary feeding Grapevine Lake.
The wastewater treatment system for this municipality consists of complete mix aeration and an average daily flow rate of 3.2 MGD. The facility is required to meet seasonal ammonia limits of 2 mg/l daily average April to Oct and a 7 day average of 5 mg/l with slightly higher limits for the winter months of 6 mg/l daily average. With those limits in place they’ve been operating, like many facilities, on a tight budget which requires extra care and time to make sure the facility is compliant.
Grab samples and composite samples are run regularly to ensure their process is working properly. Process control samples are taken at the end of the aeration system for solids, NH4-N and others to make sure biological processes are functioning as they should.
One of the facility’s largest operational expenses is aeration to drive biological reduction of ammonia through Nitrification. The facility has traditionally used online Oxidation Reduction Potential (ORP) and Dissolved Oxygen (DO) as surrogate measurements for monitoring ammonia removal in the aeration basins.
The Solution
The Grapevine treatment works decided to trial the new Hach NH6000sc ammonia analyzer in one of their three aeration trains. The new analyzer and its filtration unit was installed at the end of the aeration train shown in image 1. This allowed the plant to benefit from real-time ammonia measurements through their SCADA system for the first time. Ammonia based aeration control is gaining popularity for the following reasons:
- Using the specific parameter to be treated, in this case ammonia, is more effective than using surrogate measurements like DO and ORP. This is in large part is due to other interferences and demands on measurements like DO and ORP. They are not direct measurements and because of it, effective direct control for nitrification is limited.
- Energy savings increase with every data quality improvement. Going from ORP testing to online DO is a substantial optimization change. Further upgrading from online DO to online ammonia measurements reduces error and improves system performance even more.
- Overtreating for ammonia removal can have significant carbon emission implications. From a macro level, running higher blower speeds and header pressures or running blowers outside of their optimal curve, etc. when it results in over treatment increases unnecessary carbon emissions. Eia.gov states in 2023 the average US CO2 emissions was .81 lbs per kWh used. Running blowers at lower speeds within their pump curves reduces wear and extends their working life.

This new analyzer brought a level of diurnal visibility to the facility which grab sampling and composite samples were lacking.
In figure 3 you can see the results of a few weeks of operation. The plant used the real-time ammonia values from the analyzer to slowly start reducing aeration. They closely monitored this third of the treatment plant and allowed the ammonia levels to increase to a level that’s still below their permit requirement.
In figure 3 the first data point for amps represents standard operation before making changes using the new analyzer. The following reductions in blower speed continues until they were able to completely shut off one of their two 100 hp blowers. They operated with one blower off manually for a day as successful test and then put the second blower back online. Overall, during this period, they saw a 28% decrease in power usage when the second blower was removed, and then an average power reduction of 18.4%. This reduction in aeration could represent an annualized savings of $29,953 based on the facility’s electrical rate.
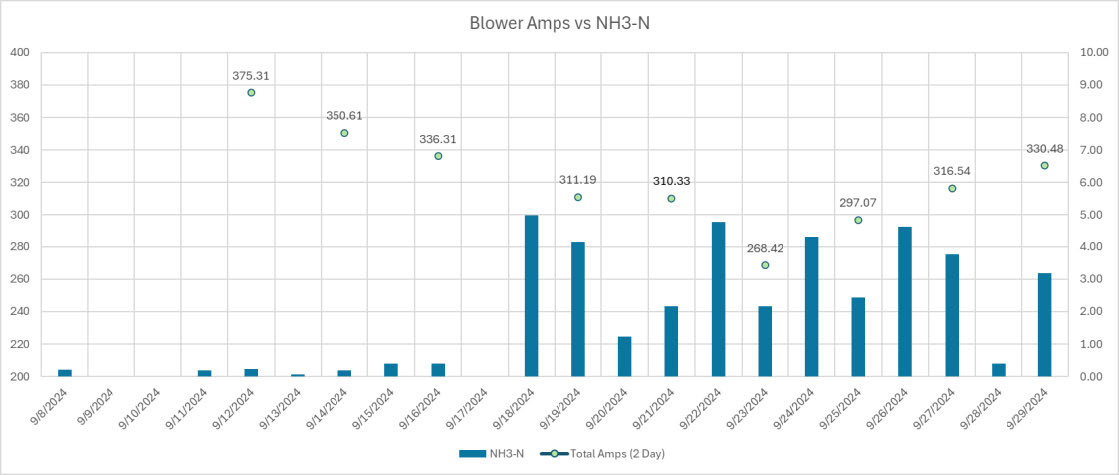
In addition to dollars saved this case could represent an annualized carbon emissions reduction of around 404,449 lbs. CO2.
Conclusion
The new Hach NH6000sc online ammonia analyzer proved to be a great tool for the city allowing for a new level of visibility that was not previously possible. The Hach gas sensing technology provides a fast response time unlike other technologies, providing values every 5 minutes for efficient control and monitoring in a fast-changing biological environment. In a quote from one of the system managers:
“The new analyzer from Hach allowed us the ability to confidently reduce aeration to optimize efficiency without worry of reaching our ammonia limit.”

The new design allows for out of the box installation outdoors standing up to the elements without a second enclosure. The new reagent set-up is used at a rate which allows for replacement once every 6 months. New sample pumping and stout air backflush has all been integrated into the unit. But often analyzers are only as accurate and easy to use as their filtration system.
The new Hach FX620 filtration systems ability to deliver heavy air backflush makes manually cleaning frequency low even in high solids applications like mixed liquor. The staff pulled the filter to inspect it and found it looking great. Plant staff was surprised by how lightweight and easy to remove the filter was. The new snap in membrane design made for quick removal and secure installation. In the end improving plant process, protecting the environment, maintaining permit requirements, and balancing it all by being good stewards of funding and energy all play a role in a decision to use online sensors and analyzers. Hach’s innovation is moving forward to bring tools like this to the field with values rooted in simplicity and accuracy.

Additional Resources
Pairing Digital Flow Sensors with the Hach AS950 Automatic Sampler
go to HACH.COMIntroduction When the Hach® AS950 Automatic Sampler launched in 2015, we were excited to offer the option to connect digital flow sensors to the controller. Digital flow sensors can be used for collecting flow proportional samples, as well as triggering...

Maximizing Phosphorus Removal
go to HACH.COMPhosphorus removal plays a critical role in protecting waterways, meeting permit requirements, and managing treatment costs. But optimizing phosphorus treatment isn’t always straightforward, especially when processes, influent conditions, and system...

Optimize Total Suspended Solids (TSS) & Turbidity Measurement
go to HACH.COMAccurate measurement of Total Suspended Solids (TSS) and turbidity is vital for effective water quality management across municipal, industrial, and wastewater environments. Real-time insights into solids concentrations empower operators to improve...
Privacy Policy | Cookie Policy | Cookie Settings | Do Not Sell or Share My Data
©Hach All rights reserved.




